4.3 Electronic Configuration
Keya Jani, Gurdit Sood
Quantum Numbers
What are quantum numbers and why are they important? A set of quantum numbers describes the position and the energy of an electron within an atom.1 There are four sets of quantum numbers: n, ℓ, mℓ, ms. They are related to each other, so they must be put into a specific order.2
What does the n quantum number mean? The n quantum number is the Principal Quantum Number and it describes the Principal Shell and helps visualize the distance of the electron from the nucleus. The n quantum number is represented by positive integers (n = 1, 2, 3,4 …); the lower the number, the closer the electron is likely to be to the nucleus. The principal shell also describes the energy of the electron. The higher the quantum number, the further the electron is from the nucleus, making it more reactive, and higher in energy.
What does the ℓ quantum number mean? The ℓ quantum number is the Orbital Angular Momentum Quantum Number and describes the sub shell (aka the shape of the orbital). The ℓ quantum number can take on any value from 0 to n-1.
Example: if the principal shell is n = 4, the subshells can be ℓ = 0, 1, 2, or 3.
What does the mℓ quantum number mean? The mℓ quantum number is the Magnetic Quantum Number and determines the orientation of the orbital. The mℓ ranges from - ℓ to + ℓ.
Example: if the subshell is 3, the orientation of the subshell can be mℓ = -3, -2, 1, 0, 1, 2, or 3.
What does the ms quantum number mean? The ms quantum number describes the spin of the electron, clockwise or counterclockwise. The ms value can either be -12 or +12
How does the l quantum number relate to orbital shapes? In the previous section, you learned about the different orbital shapes. The ℓ quantum number determines the shape.
Notation:
- ℓ = 0 — s shape orbital
- ℓ = 1 — p shape orbital
- ℓ = 2 — d shape orbital
- ℓ = 3 — f shape orbital
Note: when naming the subshell, you also have to consider the principal quantum. This is done by writing the principal quantum number and then the orbital shape.
Example:
- If n = 3 and ℓ = 2, the subshell name is 3d
- If n = 2 and ℓ = 1, the subshell name is 2p
- If n = 4 and ℓ = 3, the subshell name is 4f
- If n = 2 and ℓ = 3, the subshell cannot exist because the ℓ quantum number has to be less than the principal quantum number.
Orbitals
What is an orbital? Orbitals are regions of high electron density, meaning that electrons are more likely to be found within the orbital.3 Orbitals are not real, but more so a mathematical explanation for where electrons are likely to be found. Think of orbitals as “electron density clouds”, as there is a high probability of electrons to be found within them. Orbitals come in different shapes, and these shapes are based upon the Orbital Angular Momentum Quantum Number (ℓ). Let’s examine the different shapes.
Recall that the subshell is dependent upon the value of the principal quantum number. When ℓ = 0, this is known as the s orbital. The s orbital is spherical in shape (Figure 1).4
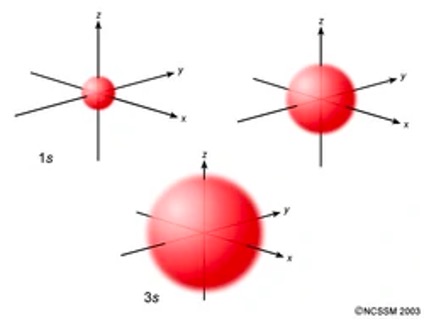
Figure 1: s-Orbitals.5
When ℓ = 1, the orbital is known as the p orbital. It looks like a dumbbell and since ℓ = 1, the Magnetic Quantum Number (mℓ) equals -1, 0, 1. The amount of values that are outputted from mℓ refers to the different orientations of that orbital. In this case, there are 3 values for mℓ, and the orbital is the p orbital, hence there will be 3 different orientations for the p orbital. Figure 2 highlights how these orientations look.
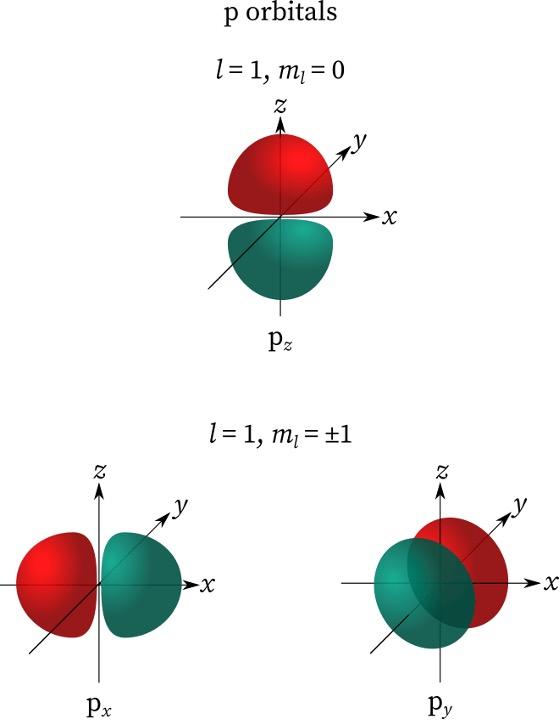
Figure 2: p-Orbitals.6
When ℓ = 2, the orbital is known as the d orbital. The values for mℓ are -2, -1, 0, 1, 2. Those are 5 possible orientations, and Figure 3 showcases the interesting shape and the 5 possible orientations of the d orbitals.
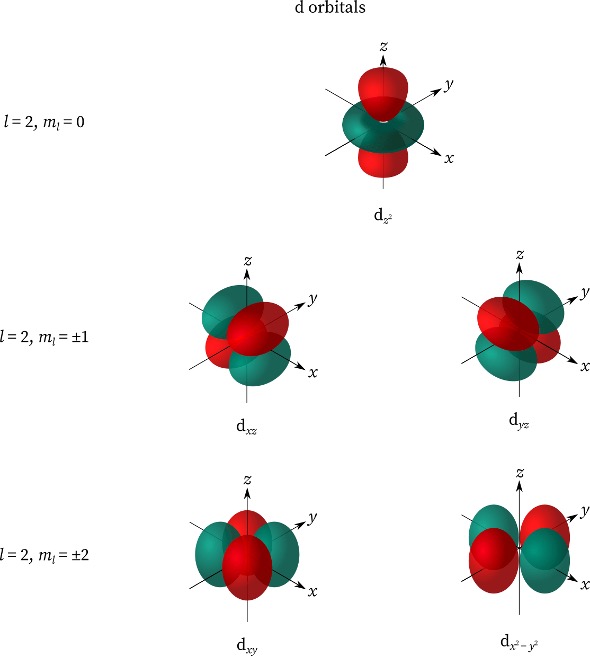
Figure 3: d-Orbitals.7
When ℓ = 3, the orbital is known as the f orbital, and the values for mℓ are -3, -2, -1, 0, 1, 2, 3. That is a total of 7 values, indicating 7 different orientations in space.
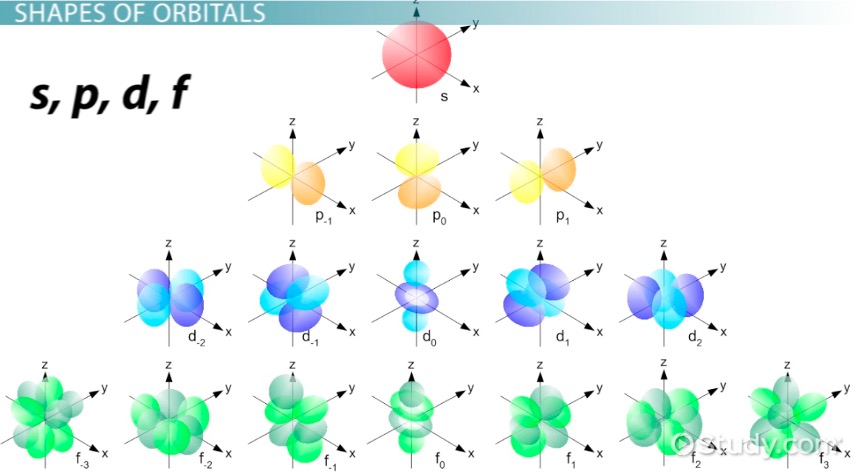
Figure 4: f-Orbitals.8
When we have orbitals, we can also have nodes, which are areas where the probability of finding an electron is 0. Planes can be used to represent where electrons are not found. These are called nodal planes (also known as angular nodes). Figure 5 highlights how angular nodes look like. Another type of node are radial nodes. These types of nodes are spherical in shape (look at Figure 6).
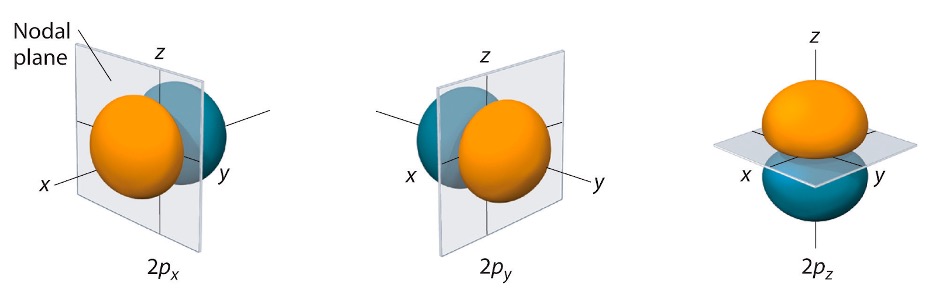
Figure 5: Nodal Planes.9
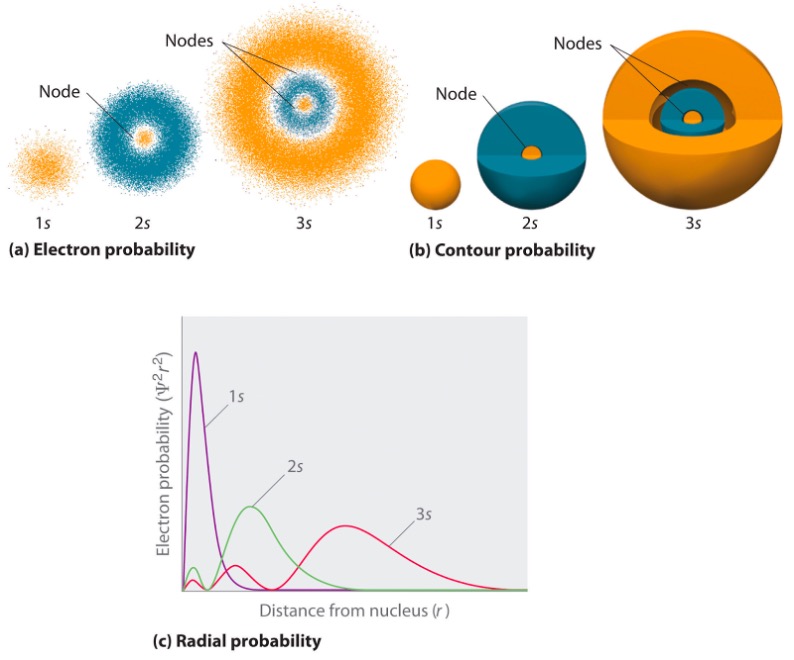
Figure 6: Radial Nodes.10
Knowing how to calculate radial nodes is quite important. The formula for calculating radial nodes is as follows:
Radial Nodes = n - ℓ - 1
Example: Determine the amount of radial nodes in a 2s orbital
Radial nodes = 2 - 0 - 1 = 1
The value for n is 2, the value for ℓ is 0 since it is an s orbital.
Where n is the Principal Quantum Number and ℓ is the Orbital Angular Momentum Quantum Number.
The Formula for determining angular nodes is quite simple, it is just the value of ℓ.
Angular Nodes = ℓ
Example: Determine the amount of angular nodes in a 2s orbital Angular nodes = 0
It’s 0 because it is an s orbital, and the value of ℓ for a s orbital is 0.
Electron Configurations
What is electron configuration? The electron configuration is essentially the description of how electrons are placed in the orbitals (seperated into the principal quantums and subshells) of an atom.
It is important to note that the total energy of an atom depends on the principal quantum, the subshells, and the electron repulsions. The electron repulsions are created when electrons are placed in particular orbitals. The atom seeks to minimize its energy, therefore, the electrons are placed within the orbitals in a particular way determined through experimentation.
How do I know the order of placing electrons into orbitals? Follow Figure 7 to determine how to fill the orbitals.
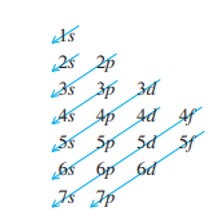
Figure 7: Orbitals In Order of Increasing Energy Level.11
The Pauli Exclusion Principle Only two electrons can occupy the same orbital, and they must have opposite spins.
Note: each orbital can support 2 electrons.
- S subshell has 1 orbital and total 2 electron capacity
- P subshell has 3 orbitals and total 6 electron capacity
- D subshell has 5 orbitals and total 10 electron capacity
Hund’s Rule Electrons must be placed alone in each orbital, and in parallel formation, before they are paired up. All electrons carry the same energy, so by spreading the electrons across the orbitals, the energy is dispersed. Moreover, parallel spins ensure the electrons are attracted to the nucleus. Both these factors minimize the total energy of the atom.
The Aufbau Principle Electron configurations of elements are related to the order of increasing atomic number. To go from one element to another, one must add protons, neutrons, and electrons to the atoms. This is represented in their electron configuration as the atomic number equals the number of electrons.
The orbitals of an atom are always filled with electrons from the orbital with the lowest energy to the one with the highest energy. Figure 7 shows the order in which orbitals of an atom are filled.
For example, consider the element Mn (manganese) with an atomic number of 25. A neutral Mn atom therefore has 25 electrons, and we start filling orbitals from 1s to the highest-energy orbital needed.
Mn: 1s2 2s2 2p6 3s2 3p6 4s2 3d5
Note that orbitals are filled from lower to higher energy levels. They are not necessarily filled from lower to higher quantum number (e.g., 4s orbital is filled before 3d orbital despite having a larger quantum number).
Important: There are exceptions to the Aufbau principle, notably Cr and Ag. Following the Aufbau principle, the configurations of these atoms would be [Ar] 4s2 3d4 and [Ar] 4s2 3d9, respectively. However, half-filled or completely-filled orbitals are more stable. Therefore, 1 electron from the 4s orbital is transferred to the d orbital for these atoms to achieve a half-filled 4s orbital and a half-filled/completely-filled 3d orbital. The actual configurations of Cr and Ag are therefore [Ar] 4s1 3d5 and [Ar] 4s1 3d10, respectively.
Practice Problems
Determine the number of angular nodes in a 2p orbital
Determine the number of radial nodes in a 4d orbital
Determine the angular and radial nodes in a 3p orbital
Determine the complete electron configuration for the element Ag
Determine the complete electron configuration for the element germanium
Determine the element with the following electron configuration: [Kr] 4d105s^{2}5p^{2}
References
Petrucci RH, Herring FG, Madura JD, Bissonnette C. General Chemistry: Principles And Modern Applications. 11th ed. Toronto, ON: Pearson Canada; 2017:337-347.
Admin. Quantum Numbers (Principal, Azimuthal, Magnetic and Spin) - Definition, Detailed Explanation, Videos and FAQs. BYJU’S. https://byjus.com/chemistry/quantum-numbers/. Published July 23, 2018. Accessed July 18, 2022.
Libretexts. Electronic orbitals. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals. Published July 14, 2020. Accessed July 18, 2022.
Petrucci RH, Herring FG, Madura JD, Bissonnette C. General Chemistry: Principles And Modern Applications. 11th ed. Toronto, ON: Pearson Canada; 2017:337-347.
s, p, d, f orbitals - chemistry: Socratic. Socratic.org. https://socratic.org/chemistry/the-electron-configuration-of-atoms/arrangement-of-electrons-in-orbitals-spd-and-f. Accessed July 16, 2022.
ChemistryGod. Magnetic Quantum Number ~ ChemistryGod. ChemistryGod. https://chemistrygod.com/magnetic-quantum-number. Published January 27, 2020. Accessed July 16, 2022.
ChemistryGod. Magnetic Quantum Number ~ ChemistryGod. ChemistryGod. https://chemistrygod.com/magnetic-quantum-number. Published January 27, 2020. Accessed July 16, 2022.
Magnetic quantum number for d orbitals. Physics Stack Exchange. https://physics.stackexchange.com/questions/292841/magnetic-quantum-number-for-d-orbitals. Accessed July 16, 2022.
What is the difference between nodal surfaces and nodal planes?: Socratic. Socratic.org. https://socratic.org/questions/569904497c01495ce7abf9ea. Published January 27, 2016. Accessed July 16, 2022.
What is the difference between nodal surfaces and nodal planes?: Socratic. Socratic.org. https://socratic.org/questions/596481c6b72cff2ef05a11f9. Published July 11, 2017. Accessed July 16, 2022.
Petrucci RH, Herring FG, Madura JD, Bissonnette C. General Chemistry: Principles And Modern Applications. 11th ed. Toronto, ON: Pearson Canada; 2017:353-363.