4.4 - Atoms and Electromagnetic Radiation
Pranay Soma, Kabishan Vinothan
The Periodic Table
The periodic table we use was originally proposed by Dmitri Mendeleev. It is ordered left to right by increasing atomic numbers. To review, the atomic number represents the number of protons in an atom. It also represents the number of electrons of a neutral atom, as # of electrons = # of protons. Additionally, the # of neutrons can be calculated by subtracting the atomic number from the atomic mass. Mendeleev also arranged the table in a unique way to emphasize the trends observed in the various groups (columns) and periods (rows). It is important to recognize that most chemical properties exist due to valence electrons. The first trend is shown in Figure 1 below. There are certain “blocks” portraying the electron configurations, which show the nature of the valence electrons (s, p, d, f). The s block (first two columns) and p block (last six columns) elements are known as the main-group elements. The d block (columns 3-12) are called the transition elements. The f block elements are taken out so the table fits properly. Fifteen elements with atomic numbers from 57 to 71 are called lanthanides, and fifteen from 89 to 103 are called actinides.1
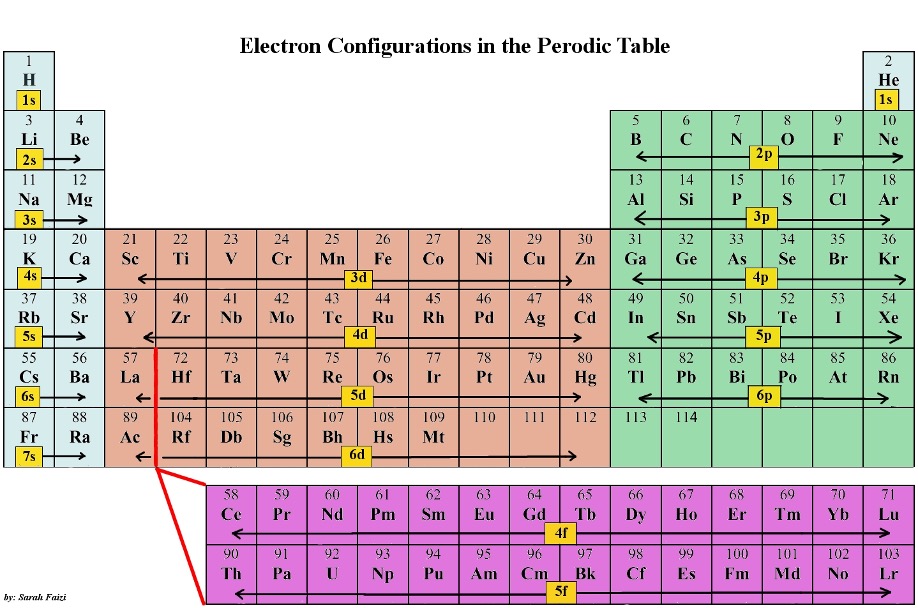
Figure 1: Periodic Table with Electron Configurations.2
Metals, Nonmetals, and Ions
All the elements in the periodic table can be categorized into two general groups: metals and nonmetals, which exhibit certain properties. Metals are usually hard, malleable and ductile. They also have pretty high electrical and thermal conductivity along with high melting and boiling points. On the other hand, nonmetals are softer, not good conductors, brittle solids, and a lot of them are gases at room temperature, indicating lower boiling points. There is also another group called the metalloids, which behave like both metals and nonmetals. On the periodic table itself, the non-metals are on the right side (with the exception of hydrogen), and the rest are metals. There is a “metalloid ladder” that also exists, which separates the two main groups as shown in Figure 2.
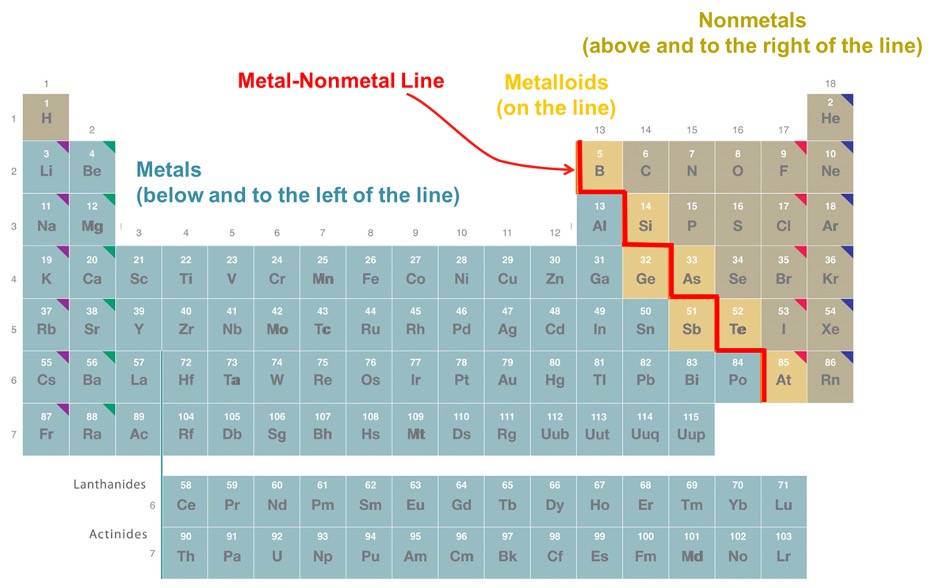
Figure 2: Periodic Table with Metallic Categories Highlighted.3
Now we will go over the electron configuration of some groups and their ions. The first is the noble gases, which is group 18. The elements in this group have the maximum number of valence electrons, and are not very reactive. The electron configuration of the outermost shell in helium is (1s2), while the rest of the noble gases are (ns2p6).
Next are the main-group metal ions, which consist of groups 1 and 2, known as the alkali metals and alkaline earth metals respectively. These are the most reactive metals, and the difference between them is their valence electron configurations being one electron apart. Group 1 is (ns1) while group 2 is (ns2), where n represents any number between 1-7 (refer to Figure 1). These metals would lose electrons to obtain the noble gas electron configurations, as losing 1 or 2 is easier than gaining 6 or 7 electrons.
The main-group nonmetal ions consist of groups 16 and 17, known as chalcogens and halogens respectively. There are the most reactive nonmetals, and the same difference of one electron lies within their designated configurations. Group 16 is (ns2 np4), while group 17 is (ns2 np5). These nonmetals would gain electrons to obtain the noble gas electron configurations, as it is more efficient.
The last group is the transition metal ions. These are groups 3 to 12 and possess the d orbital. Their ions all have charges equal to or greater than 2+. However, 2 of their valence electrons are in the (ns) orbital, while the rest are in the (n-1) d orbitals. It is important to remember that when a transition metal atom becomes an ion, it loses electrons from the (ns) orbital first. An example to illustrate this is Fe2+, which has the ground-state electron configuration of [Ar] 3d6. Two electrons are lost from the (ns) orbitals, which is why it is omitted from the electron configuration notation.
One last point to emphasize is the hydrogen atom. Despite H being a nonmetal, it is located on the left side of the periodic table. This is because its ground-state electron configuration is (ns1), just like the rest of group 1. You could consider it a halogen as it only needs one electron to obtain a noble gas electron configuration, but it does not possess the properties of the other halogens. One example is H2 is a reducing agent, while F2 and Cl2 are oxidizing agents.1
Atom and Ion Size
Atom radius is hard to define, so there is no consistent definition. This is because orbitals can extend to infinity, so despite the probability of an electron being found very far from the nucleus being low, it still exists. This means there is no absolute enclosed space around an atom. However, three types of radii are defined for empirical purposes: covalent, metallic, and ionic. The covalent radius is half the distance between two identical, covalently-bonded atoms. The metallic radius is half the distance between two identical atoms in a crystalline solid metal. The ionic radius is the distance between the nuclei of the ions in an ionic bond.
There are two main trends regarding atomic radius on the periodic table. The first is that more shells in an atom results in a larger radius. Therefore as you go down a group in the table, the atomic radius increases. The second trend is that atomic radius decreases with a higher effective nuclear charge (Zeff). Zeff represents the amount of positive charge an electron “feels”. This is important because in an atom, the core electrons (electrons located in the inner shells) “shield” the valence electrons from feeling the full nuclear charge. There is repulsion between the inner electrons and valence electrons. This is significant because that means there is less attraction; there is less of a pull towards the nucleus for the valence electrons. The equation that describes this Zeff = Z - S, where Z is the number of protons, and S is the number of core electrons. As the number of electrons increases, Zeff decreases, and vice versa. Now we chose the changing variable to be the number of electrons for a reason: when comparing atoms in a period. As you go right across the period in the periodic table, the number of core electrons always stays the same in that period, but the valence electrons increase. Therefore using the trend explained, as you go right across the period, the atomic radius decreases.
An ion is an atom that gains or loses electrons. Metals typically lose electrons to become a positively charged ion (known as a cation), and nonmetals typically gain electrons to become a negatively charged ion (known as an anion). In a cation, the loss of one or more electrons causes the nucleus to pull the electrons closer, resulting in the cation being smaller than the original atom. This is because the magnitude of the effective nuclear charge is greater than the electron’s negative charge since one or more electrons were lost. When comparing isoelectronic cations (ions that have the same amount of electrons in the same configuration), the ion with a greater positive charge will be the smaller ion. For instance, when we compare Na+ and Mg2+ (Figure 3), the magnesium ion is smaller as it has a greater positive nuclear charge (magnesium has more protons than sodium). On the other hand, when nonmetals gain electrons to become an anion, the anion is larger than its normal atom. This is because the addition of electrons causes the atom to hold these electrons more loosely as the effective nuclear charge remains constant. Also, since there is a greater amount of electrons, there is correspondingly a greater amount of repulsion among the electrons, causing them to spread further apart. When comparing isoelectronic anions (Figure 3), the more negatively charged anion is greater in size (greater repulsion and more loosely held).1
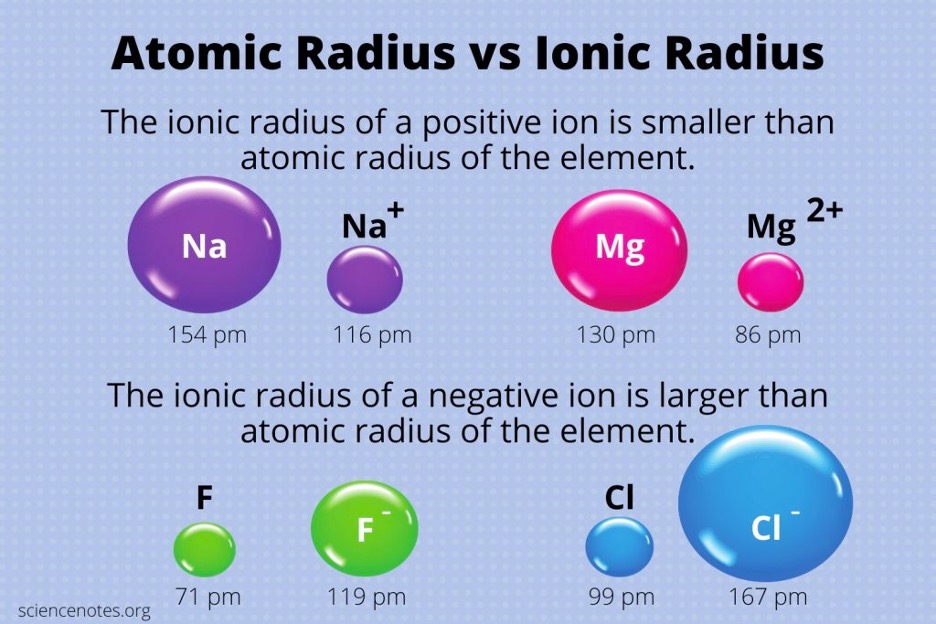
Figure 3: Summary of Atomic and Ionic Radii.4
Ionization Energy
Ionization energy describes the amount of energy that is needed in order to remove an electron from an atom at a gaseous state. Typically, this is found in metals as they lose electrons to become ions. The energy that is needed to remove this electron is known as ionization energy. For instance, the ionization energy to remove an electron from magnesium (Mg (g)) to form Mg+ is about 738 kJ/mol. The ionization energy needed to then remove another electron from Mg+ to form Mg2+ is about 1451 kJ/mol. Through these values, we can note that less energy is needed to remove the first electron than the second electron. This is because of the effective nuclear charge (Zeff). The effective nuclear charge is the amount of attractive positive charge that an electron experiences due to the protons in the nucleus. 5 When we remove the first electron from magnesium, there is now a stronger attractive positive charge on the second electron, resulting in larger ionization energy. Along with the effective nuclear charge, the atomic radii also affect the ionization energy. As the atomic radii increase, the ionization energies decrease as the electron is further from the nucleus (Figure 4). Therefore, the general trend is that ionIzation energy increases as we go left to right and bottom to top on the periodic table.1
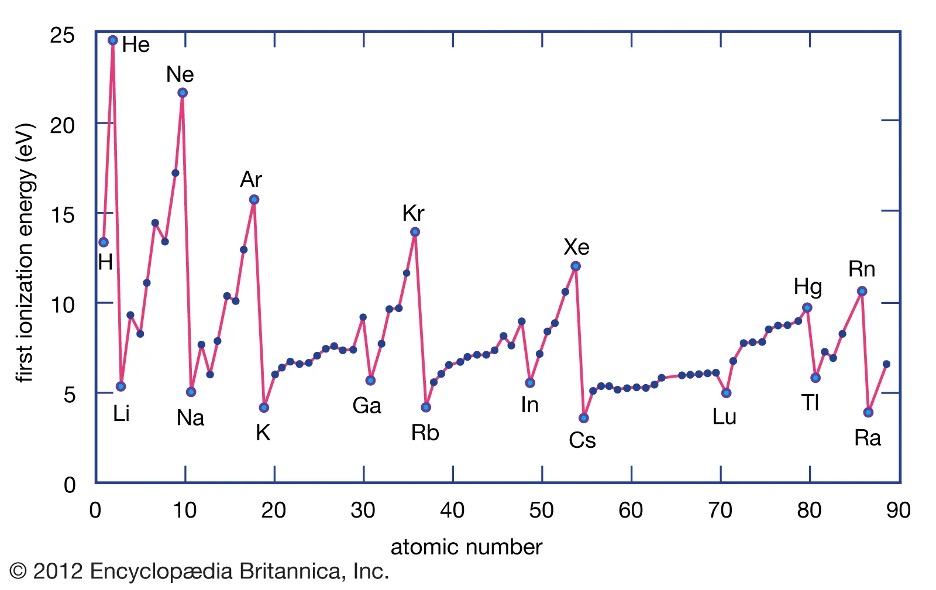
Figure 4: First Ionization Energies Compared to Atomic Number.6
Electron Affinity
In contrast to ionization energy, electron affinity (EA) describes the amount of energy released when an atom gains an electron in its gaseous state. A more negative EA value represents a higher electron affinity. This is also indicative of having a stronger attraction to the nucleus. For instance, if we look at the element chlorine (Cl), it has an electron affinity value of about -349 kJ/mol. The negative value represents an exothermic process where the energy is released. In other words, when chlorine gains an electron to become a chloride ion (Cl-), it releases energy. The general periodic table trend is that electron affinity decreases right to left and top to bottom (Figure 5). Likewise to ionization energy, this trend is also influenced by the effective nuclear charge (Zeff) and atomic radii. A greater nuclear effective charge suggests that there is a stronger attractive positive charge. This stronger attractive positive charge increases an atom’s affinity to gain an electron. Similarly, as atomic radii increases, there is a lower affinity for electrons, as the electron that would be gained will be placed into larger and further orbitals. This further distance will cause a weaker attractive force towards the nucleus.
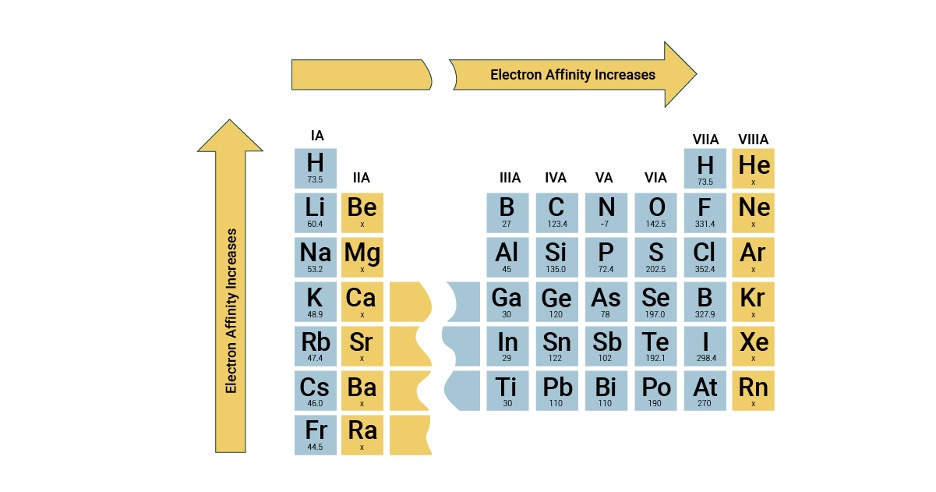
Figure 5: Trends of Electron Affinity in the Periodic Table.6
Practice Problems
- Name the element located in group 14 period 4.
- What is the valence electron configuration of Phosphorus?
- Determine the effective nuclear charge of Magnesium.
- List the following elements from least to greatest first ionization energy: Mg, O, Sr, F
- List the following elements from least to greatest electron affinity: Ca, F, Br, K
References
Petrucci RH, Herring FG, Madura JD, Bissonnette C. General Chemistry: Principles And Modern Applications. 11th ed. Toronto, ON: Pearson Canada; 2017:377-399.
Faizi S. 2.4 Electron Configurations. Chemistry LibreTexts. https://chem.libretexts.org/Courses/Valley_City_State_University/Chem_115/Chapter2%3A_Atomic_Structure/2.4_Electron_Configurations. Published 2019. Accessed July 6, 2022.
Contakes S. 8.1.1.1: The metal-nonmetal-metalloid distinction and the metal-nonmetal “line” are useful for thinking about trends in elements’ physical properties. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_%28Miessler_Fischer_Tarr%29/08%3A_Chemistry_of_the_Main_Group_Elements/8.01%3A_General_Trends_in_Main_Group_Chemistry/8.1.01%3A_The_Periodic_Table_is_an_Organizing_Concept_in_Main_Group_Chemistry/8.1.1.01%3A_The_metal-nonmetal-metalloid_distinction_and_the_metal-nonmetal_line_are_useful_for_thinking_about_trends_in_elements%27_physical_properties. Published 2022. Accessed July 7, 2022.
Helmenstine A. Atomic Radius and Ionic Radius. Science Notes and Projects. https://sciencenotes.org/atomic-radius-and-ionic-radius/. Published 2022. Accessed July 10, 2022.
V Eames E, Haas K. 1.1.2: Effective Nuclear Charge. Chemistry LibreTexts. https://chem.libretexts.org/Courses/Saint_Marys_College_Notre_Dame_IN/CHEM_342%3A_Bio-inorganic_Chemistry/Readings/Week_1%3A_Analysis_of_Periodic_Trends/1.1%3A_Concepts_and_principles_that_explain_periodic_trends/1.1.2%3A_Effective_Nuclear_Charge. Published 2022. Accessed September 2, 2022.
Ionization energy | definition & facts | britannica. Accessed July 10, 2022. https://www.britannica.com/science/ionization-energy
Electron affinity of the elements. Accessed July 10, 2022. https://www.breakingatom.com/learn-the-periodic-table/electron-affinity-of-the-elements