2.1 Practice problem answers
Pranay Soma, Gurdit Sood
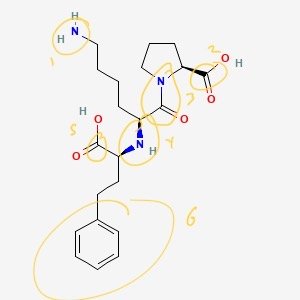
- There are six atoms that are not carbon or hydrogen.
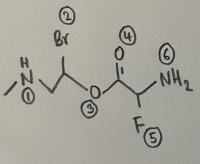
- Answer:
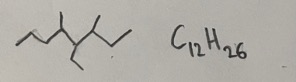
- Answer:
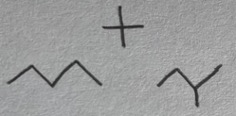
Pentane, 2-methylbutane, 2,2-dimethylpropane
4-ethyl-4,6-dimethylnonane
Answer:
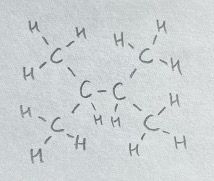
i) and ii) are constitutional isomers, as their formulas are and have different connectivities. iii) and iv) are not isomers because they are the same molecule drawn in two ways.
There are 6 functional groups in total: two amines, two esters, one amide, and one arene.
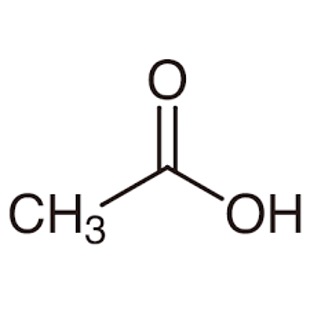
- Acetic acid is the common name for ethanoic acid. It is important to get familiar with common names since they can be tested as well.
- Alcohols contain a hydroxyl group, which allows for hydrogen bonding. This means the molecule has stronger intermolecular forces, and more energy is needed to overcome them. Therefore, it has a higher boiling point compared to hydrocarbons, which only consist of carbons and hydrogens.