2.1 Structures of Organic Compounds
Pranay Soma, Gurdit Sood
Introduction to Organic Compounds
Previously, organic compounds were thought to be only created by living organisms, hence the term “organic”. However, this was soon proven to be false, as it was discovered that they can be generated from inorganic compounds as well. Despite this, the term “organic” stuck, and organic compounds are usually regarded as compounds that consist of carbon and hydrogen atoms. Many organic compounds also exist with heteroatoms, which are defined as atoms that are not carbon or hydrogen. Two common examples of heteroatoms are oxygen and nitrogen.1
Structures and Representations of Molecules
Molecules can be represented in many different ways. The first method of representing a molecule is the molecular formula. Let’s look at methane for example. This contains 1 carbon atom, and 4 hydrogen atoms, hence leading to the formula; . If we wanted to represent this in a more visual sense, we can do a structural formula, which looks like this:1
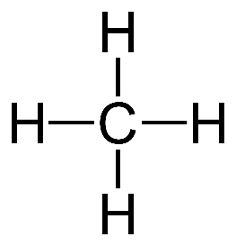
Figure 1: Structural Formula of Methane2
A useful representation for molecules when they start to become quite large is the condensed structural formula. Let’s look at propane for example. It has a molecular formula of . If we wanted to use the condensed structural formula, it would look like this: . If we look at the structural formula, we see that the condensed structural formula serves as a shortcut to see the connectivity in the molecule. The middle carbon for example, has 2 hydrogen atoms bonded to it, and we see that through both the condensed structural formula and the structural formula.
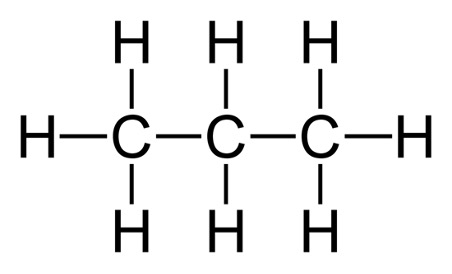
Figure 2: Structural Formula of Propane3
When carbon is bonded to 4 atoms/chains/group (which will be referred to as substituents), the carbon arranges itself in a tetrahedral structure with the different substituents bonded to it. This is due to the VSEPR theory (more on this later), but for now, just know that this is done to minimize electron repulsion.
As a result of this, the carbon can have 2 substituents that are not in the plane of the page. One would be facing out of the page and pointing right at you, and this is represented by the dark solid wedge. The other substituent, would be facing into the page, away from you, and this is represented by the dashed wedge. This type of visualization is known as a dashed-wedged line structure.
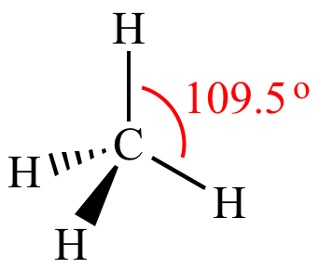
Figure 3: Dashed-Wedged Line Structure of Methane4
Nomenclature of Organic Compounds
Alkanes
To begin, let’s look at the simplest compounds: alkanes. These are hydrocarbons (compounds containing only hydrogen and carbon) that contain single bonds. The nomenclature for alkanes serves as a foundation for naming more complex molecules. For example, the compound methane, means just 1 carbon with 4 hydrogen atoms bonded to it. Methanol is like methane, but, instead of the molecule having 4 hydrogen atoms like methane, it has one hydroxyl group (), making the molecular formula become .
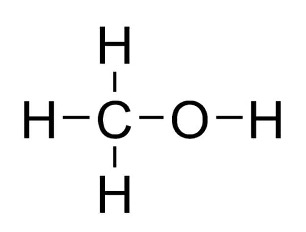
Figure 4: Structural Formula of Methanol6
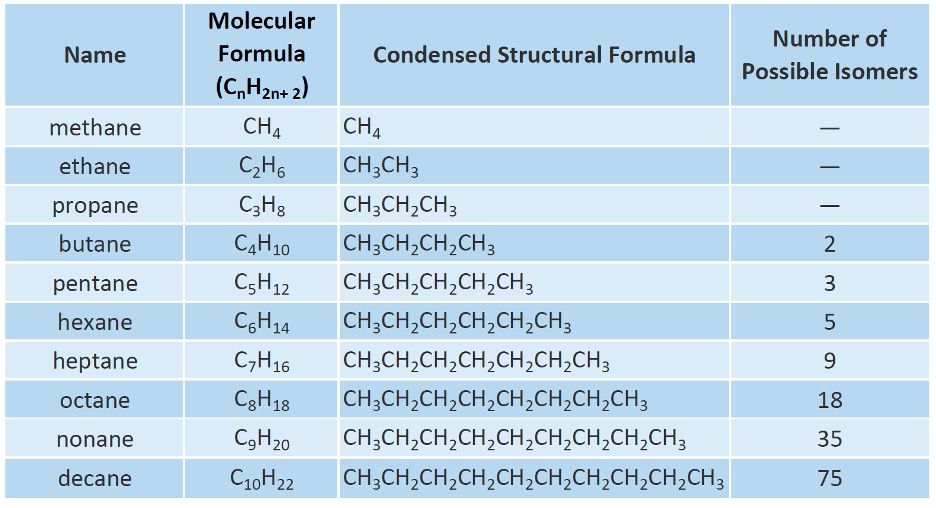
Table 1: Alkanes7
Constitutional Isomers
Now with alkanes, there are also constitutional isomers. Constitutional isomers are molecules that have the exact same molecular formula, but they differ in the connectivity of the atoms. Before we dive into understanding this concept, we need to understand another representation for molecules: the line angle formula or line structures. This visualization is quite convenient, as you don’t have to draw out hydrogens for every carbon atom. For example, the line structure for butane would look like this:
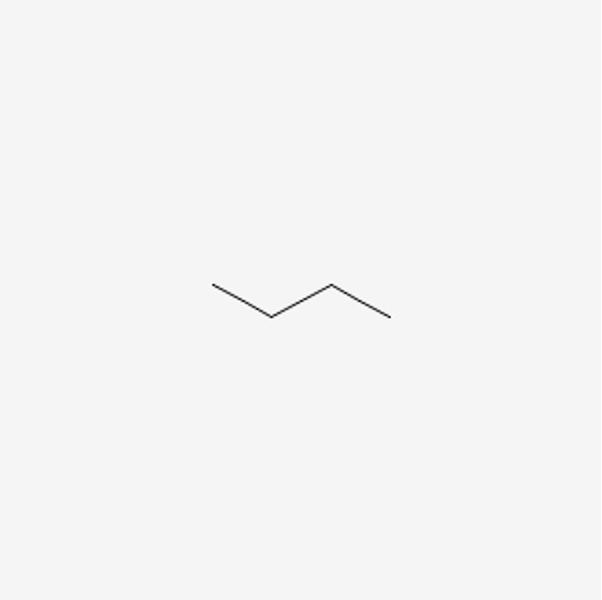
Figure 5: Line Structure of Butane8
Every end, and every corner is a carbon, and the hydrogens bonded to it is implied. Let’s take a look at an annotated example of this drawing:
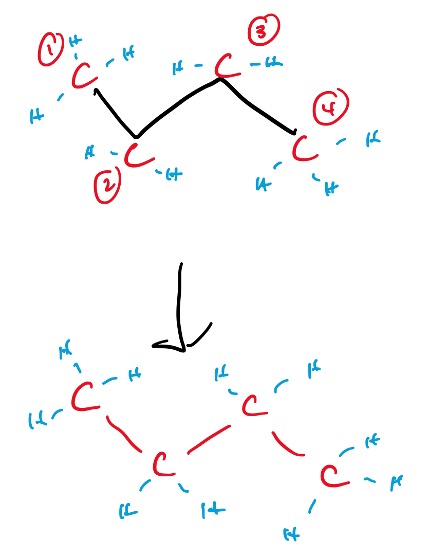
Figure 6: Annotated Line Structure of Butane
As you can see, the line structure basically conveys the needed information in a more efficient manner. The carbons are indicated in red, and their associated hydrogens are in blue. Hydrogens do not need to be drawn out in a line structure, no matter the amount of hydrogens present on the carbon.
Now that we have some understanding of a line structure, let’s look at the example of 2-methylpropane and hexane in order to understand constitutional isomers.
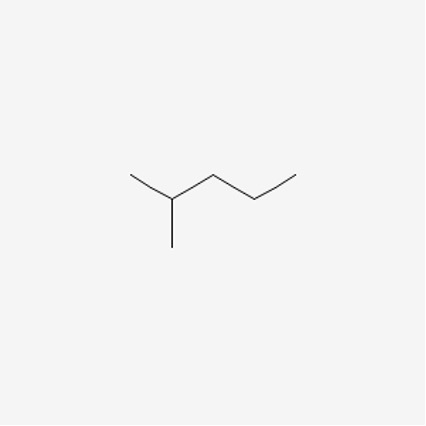
Figure 7: 2-methylpentane9
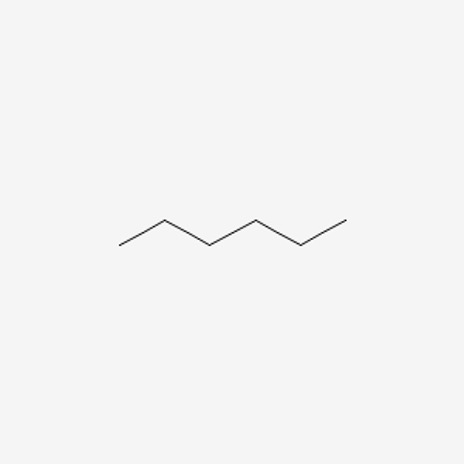
Figure 8: Hexane10
When we count the total number of carbons and hydrogens, both molecules have the same amount. We know that these are different compounds, but what makes them different? Well, their connectivity. In the first molecule, we see that the pentane molecule has a methyl group attached onto the second carbon, whereas hexane is a chain.
Anytime you have a main chain, and then a side branch attached to it, that side chain is named based on the number of carbons and has the suffix “yl” added to it. So if some molecule had a ethane side branch on it, meaning 2 carbons as a side branch from the main chain, it would be known as ethyl. Refer to Table 1 and simply add a “yl” ending for naming a branch. This is explored further in the next section.
Going back to the topic of discussion, the two molecules are called constitutional isomers because they have the same amount of material (same constitution) but just a different connectivity of the atoms. This difference in connectivity creates a difference in physical properties, which can be observed with melting and boiling points. 5
Naming Molecules
When naming molecules, having a consistent naming convention is useful for scientists to communicate what molecules they are talking about. Here are the following guidelines you should adhere to:
- When you first look at a molecule, find the longest chain. So let’s take a look at this example here, if we try to find the longest chain, it may take us a few tries, but generally, start from the most substituted side. The reason is we always want to give the branches the lowest number possible.
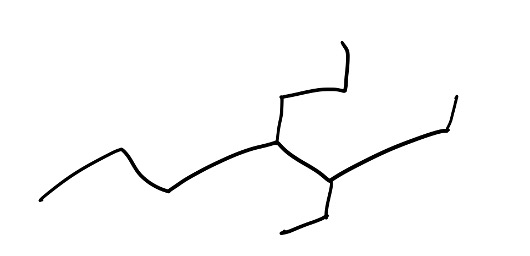
Figure 9: Example Structure
Now, here are the acceptable routes you could have taken when numbering this molecule:
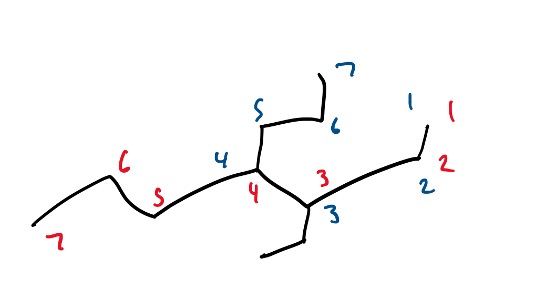
Figure 10: Example Structure with possible routes for naming the branch.
Note: always remember that in a molecule with multiple different branches or functional groups (explored in next section), it is important to give the higher priority branch or functional group the lowest numbering.
Every branch is an alkyl group, where every branch is just a name based on the number of carbons but you add a “yl” suffix to it.
When we have multiple of the same branches, we have to add the appropriate prefix. So if we had a molecule with 2 methyl groups on carbons 2 and 3 on let’s say, pentane, it would be 2,3-dimethylpentane.
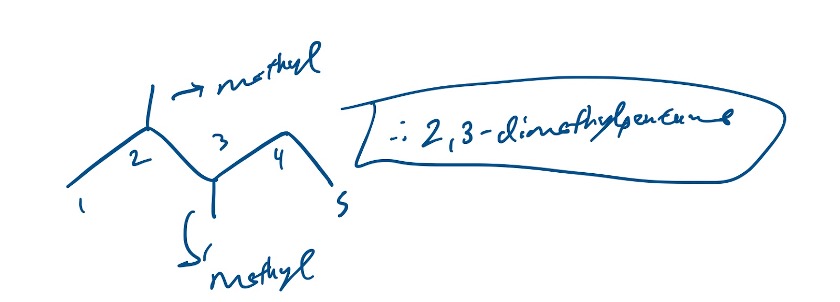
Figure 11: 2,3-dimethylheptane
So with the molecule we were discussing, let’s name the side branches and see what we are working with.
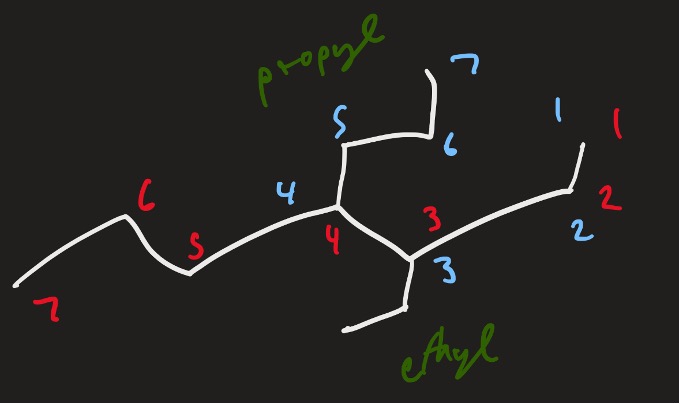
Figure 12: Example structure with named branches
- Lastly, when naming, make sure to
- Separate any numbers with commons among other numbers, and use hyphens when separating numbers from letters.
- When naming the molecule with the different substituents, make sure to do it alphabetically.
Thus, the name of this molecule would be: 3-ethyl-4-propylheptane.
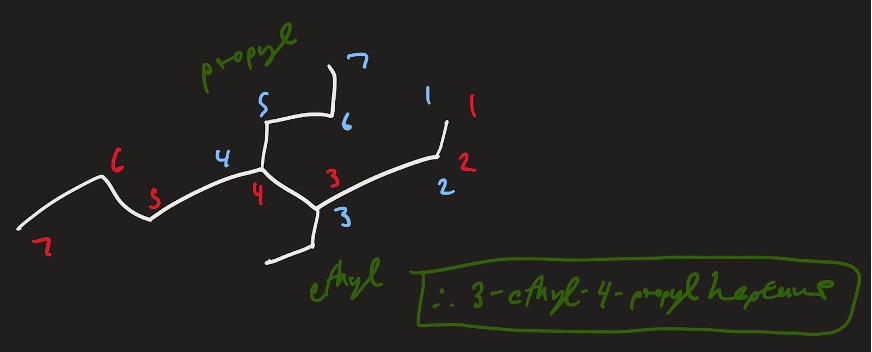
Figure 13: 3-ethyl-4-propylheptane
Functional Groups
A functional group is a group of atoms that possess unique chemical properties (e.g. boiling point) due to their connectivity. This allows them to undergo specific reactions through distinct mechanisms. In this course, there are 15 functional groups you should be familiar with. Please refer to Table 2 below, which contains 2 extra functional groups you do not need to know.
Starting off with the alkanes, these are the simplest functional groups, consisting of only single bonded carbons and hydrogens. Next are alkenes, which are similar to alkanes, but differ in the fact that they contain at least one carbon-carbon double bond.
Finally, we have alkynes, which are also similar to alkanes, but contain at least one carbon-carbon triple bond. All three of these fall under the category known as hydrocarbons, which refers to compounds that consist entirely of carbons and hydrogens.
Next are alkyl halides, which are alkyl branches with a halogen attached to them. When naming the halide part, you must use their designated branched names. So F is “fluoro”, Cl is “chloro”, Br is “bromo” and I is “iodo”. The rest of the nomenclature rules are the same.
Alcohols are alkyl branches with an hydroxyl group attached to them. A hydroxyl group is an oxygen and hydrogen bonded together. When naming alcohols, the only thing that changes is the suffix, where “ane” becomes “ol”.
An ether is simply an oxygen attached to two alkyl groups. When naming ethers, you first insert the term “oxy”. Then, on the left side of the term, you name the lowest priority group, and the highest priority group on the right side. Note that priority can refer to the number of carbon atoms in a branch (more carbon atoms = higher priority), as well as the presence of higher priority functional groups, which will be explained later in this section.
Amines are alkyl groups attached to a basic nitrogen atom with a lone pair. They are often seen as derivatives of ammonia, as the hydrogens are replaced by the alkyl groups. Naming them is similar to alkyl halides, where you use the designated branched name “amino”.
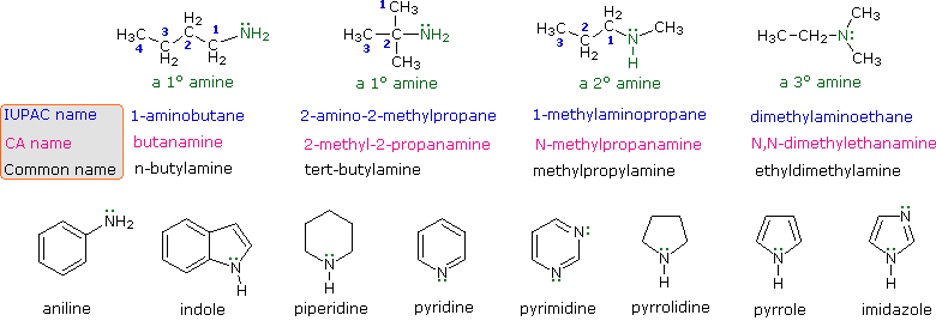
Figure 14: Different ways to name amines11
Now let’s go over functional groups that contain a carbonyl group, which is a carbon double bonded to an oxygen.
The simplest of these are aldehydes, which is a carbonyl group attached to an alkyl group and a hydrogen. When naming aldehydes, the suffix is “al”.
Next are ketones, which is a carbonyl group attached to two alkyl groups. The suffix for ketones is “one”.
A carboxylic acid is a carbonyl group attached to an alkyl group and a hydroxyl group. When naming, you must use the suffix “oic”, and you add “acid” as another word at the end.
An ester is a carbonyl group attached to an alkyl group and an oxygen that is connected to another alkyl group. Naming of esters is unique as it has two words describing two parts of the chain. The first word describes the alkyl branch connected to the O, while the second word describes the alkyl group connected to the carbonyl group. The suffix of the second word is also changed to “oate”.
The final group is an amide, which is a carbonyl attached to an alkyl group and a nitrogen atom. When naming, the suffix is “amide”.
There are three groups left to cover, and they are similar in that they possess a benzene ring. The first are the arenes, which is a benzene attached to an alkyl group. The suffix when naming is “benzene”.
Next are aryl halides, which is a benzene attached to a halide. The suffix is “benzene” too, with the prefix being the designated branched halogen names mentioned earlier.
The third and final group are the phenols, which is a benzene attached to a hydroxyl group. The suffix when naming is “phenol”.
There are a lot of rules when it comes to naming these functional groups, but it is important that we can consistently differentiate between all these complex molecules. The best way to remember everything is to get familiar with them through practice.
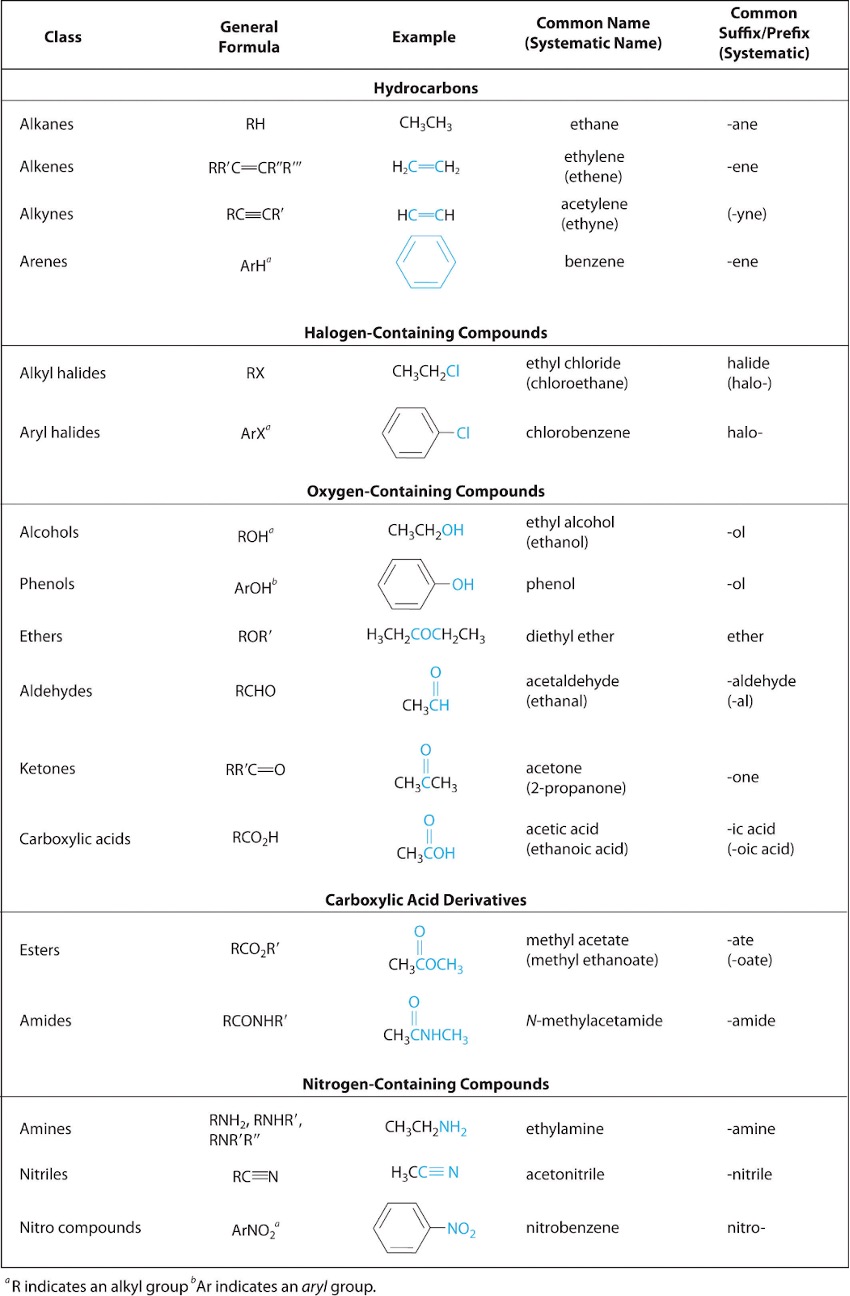
Table 2: List of 17 functional groups12
Practice Problems
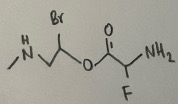
- How many heteroatoms are there in the following compound?
Write the molecular formula and line formula for 5-ethyl-4,6-dimethyloctane.
Draw all the possible constitutional isomers of .
Write the IUPAC names for the isomers of .
Name the following alkane:
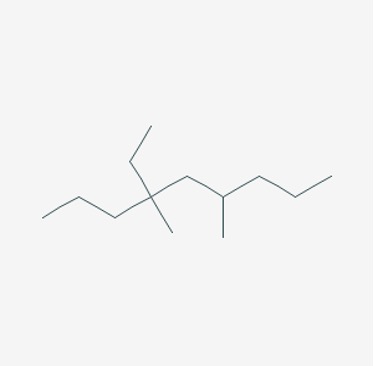
Figure 15: An alkane13
Draw the structural formula for 2,3-dimethylbutane.
Which of the following compounds are constitutional isomers?
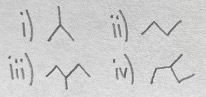
- Identify the functional groups in lisinopril:
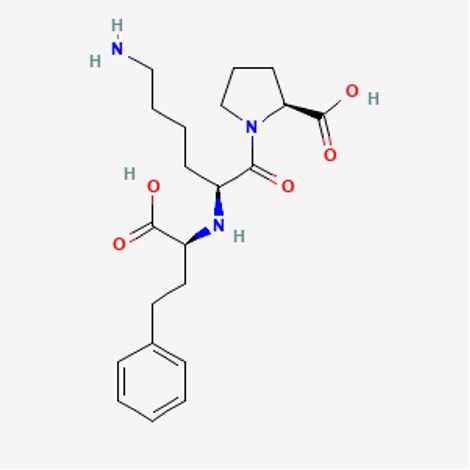
Figure 16: Lisinopril14
Draw the structure of acetic acid.
Alcohols are observed to have higher boiling points than hydrocarbons of similar molar mass. Why might this be?
References
- Petrucci RH, Herring FG, Madura JD, Bissonnette C. General Chemistry: Principles And Modern Applications. 11th ed. Toronto, ON: Pearson Canada; 2017:1208-1214.
- Sharma, A., 2016. What is the structural formula for methane? | Socratic. [online] Socratic.org. Available at: https://socratic.org/questions/what-is-the-structural-formula-for-methane. Accessed 27 June 2022].
- Propane Formula - Check Chemical and Structural Formula of Propane. BYJUS. https://byjus.com/propane-formula/. Published 2022. Accessed June 27, 2022.
- Illustrated Glossary of Organic Chemistry - VSEPR. Chem.ucla.edu. http://www.chem.ucla.edu/~harding/IGOC/V/vsepr.html. Accessed June 27, 2022.
- Petrucci, R. H., Herring, F. G., Madura, J. D., & Bissonnette, C. (2017). Names and Formulas of Organic Compounds. In General Chemistry: Principles and Modern Applications (Eleventh, p. 94-98). essay, Pearson.
- Methanol: Definition, Formula, Structure and Uses | Biology Dictionary. Biology Dictionary. https://biologydictionary.net/methanol/. Published 2017. Accessed June 27, 2022.
- Chapter 7: Alkanes and Halogenated Hydrocarbons. https://wou.edu/. https://wou.edu/chemistry/courses/online-chemistry-textbooks/ch105-consumer-chemistry/ch105-chapter-7/. Published 2022. Accessed June 27, 2022.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7843, Butane. https://pubchem.ncbi.nlm.nih.gov/compound/Butane. Accessed June 27, 2022.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7892, 2-Methylpentane. https://pubchem.ncbi.nlm.nih.gov/compound/2-Methylpentane. Accessed June 27, 2022.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 8058, Hexane. https://pubchem.ncbi.nlm.nih.gov/compound/Hexane. Accessed June 27, 2022.
- Reusch W, Emeritus. Nomenclature of Amines. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_%28Organic_Chemistry%29/Amines/Nomenclature_of_Amines. Published 2020. Accessed June 27, 2022.
- Functional Groups and Classes of Organic Compounds. Saylordotorg.github.io. https://saylordotorg.github.io/text_general-chemistry-principles-patterns-and-applications-v1.0/s28-01-functional-groups-and-classes-.html. Published 2022. Accessed June 27, 2022.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 57491025, 4-Ethyl-4,6-dimethylnonane. https://pubchem.ncbi.nlm.nih.gov/compound/4-Ethyl-4_6-dimethylnonane. Accessed June 27, 2022.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5362119, Lisinopril. https://pubchem.ncbi.nlm.nih.gov/compound/Lisinopril. Accessed June 27, 2022.