2.2 Alkanes and Cycloalkanes
On this page
Gurdit Sood
Alkanes
An interesting thing about alkanes is that within C-C bonds, there is free rotation, which can lead to different conformations of a molecule. A conformation is the same molecule, but due to the rotation within the C-C bond, the placement of the substituents in space on one of the carbons will be different from the placement before the rotation. It is important to note that rotation around the C-C bond is a physical change and different conformations are the same molecule with the same properties.1
Newman Projection
One good way to understand how these rotations work is to look at Newman projections. To understand how Newman projections work, let’s look at ethane. When looking down on the bond of the molecule (so that the two carbons are aligned and C2 is in the back), we can see the substituents of that back carbon, and the substituents of the front carbon. We can see it in three ways, where they could be perfectly aligned with each other (eclipsed), opposite to each other (staggered, as shown in Figure 1 below), or perhaps close to each other (eclipsed).
To draw a Newman projection, the carbon upon which you are looking on (C1) will be drawn as an upside down “y”. This will show the substituents bonded to that front carbon. The back carbon (C2), which is invisible to you as you look down on the first carbon, is drawn as a circle, with lines sticking out that represent the shape of “y” as well.
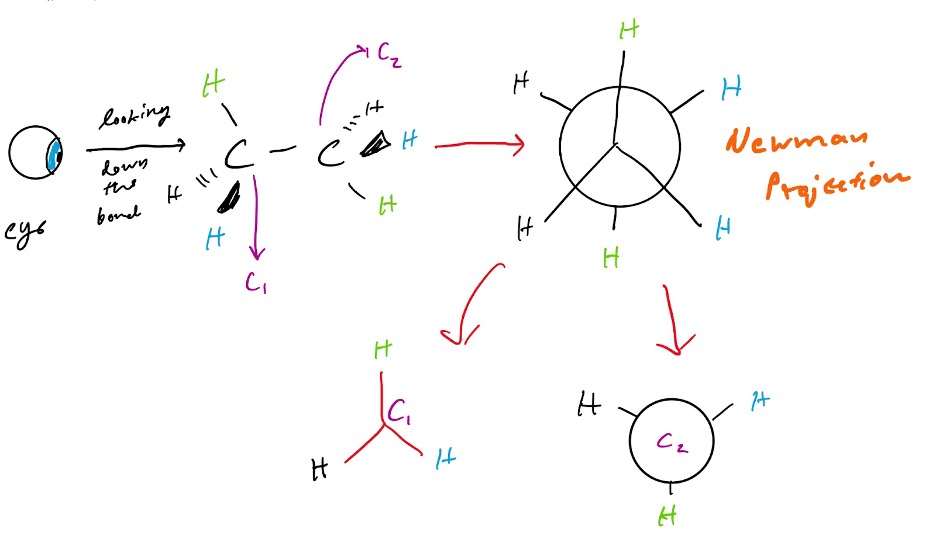
Figure 1: Newman Projection for Ethane (Staggered)
Now, Newman projections help us understand different conformations based on different rotations. When taking a look at the different Newman projections for Butane for example, some conformations are more stable than others. This is due to electron repulsion. If the substituents of both carbons in the C-C bond are perfectly aligned or very closely aligned, there will be high electron repulsion between those groups. This means the conformation is high in energy, making it less stable.
Now with these alignments that are high in energy, there comes some variation. With synperiplanar, the same substituents of both carbons are almost perfectly overlapping each other, making this highly unstable and hence, a lot of energy.
In a gauche conformation, the same substituents are close to each other and well spaced out, but they are not overlapping in any way. There is a decent amount of space to reduce the repulsions and hence making it more stable.
With an eclipsed conformation, the same substituents are not as close to each other, but they are close to other substituents (so a methyl group is close to a hydrogen atom on the other carbon atom for instance). There is stronger repulsion than in the gauche conformation, making this higher in energy. Figure 2 perfectly summarizes this explanation.
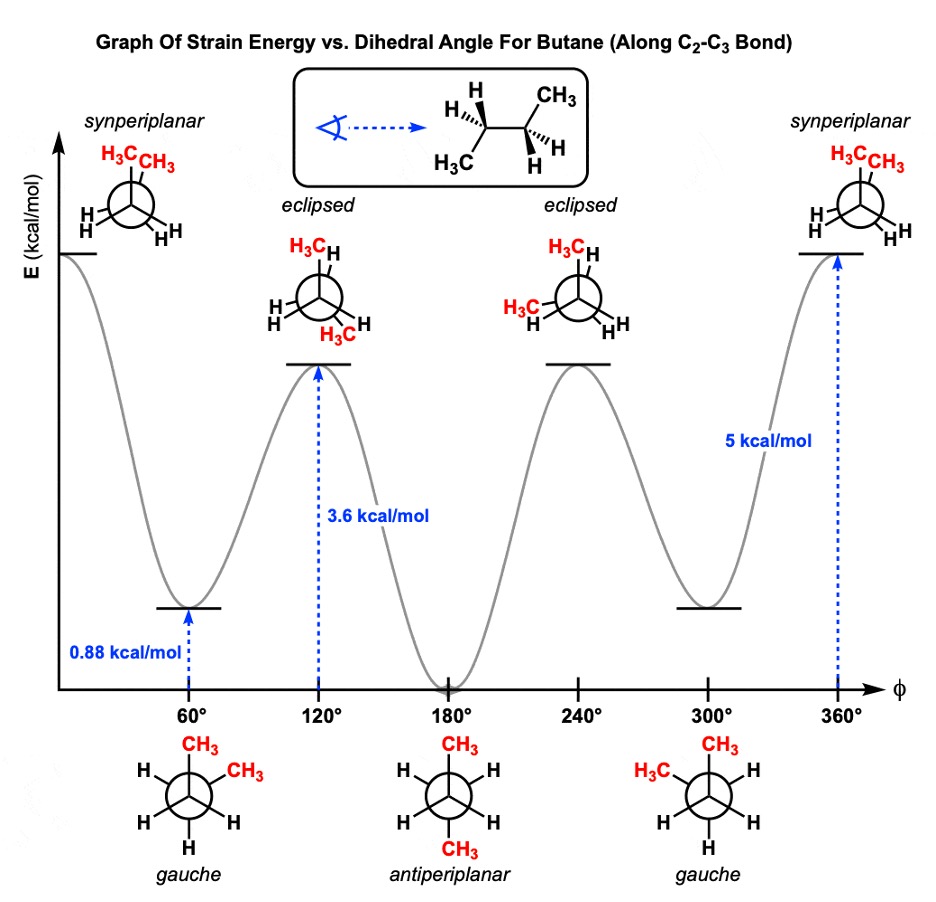
Figure 2: Newman projection for Butane2
Cycloalkanes
With alkanes, there is a general formula that dictates how many hydrogens are present based on the number of carbons:
With cycloalkanes, the formula is:
With alkanes, there is rotation around the C-C bonds and many conformations can occur. With cycloalkanes, there is not much rotation around the C-C bonds, and they are not isomers of alkanes - so cyclobutane is not an isomer of butane for example.
An important cycloalkane you should know about are cyclohexanes. Cyclohexanes have different conformations, including the boat conformation and the chair conformation. Just know that the boat conformation is less stable than the chair conformation because the substituents are closer to each other.1
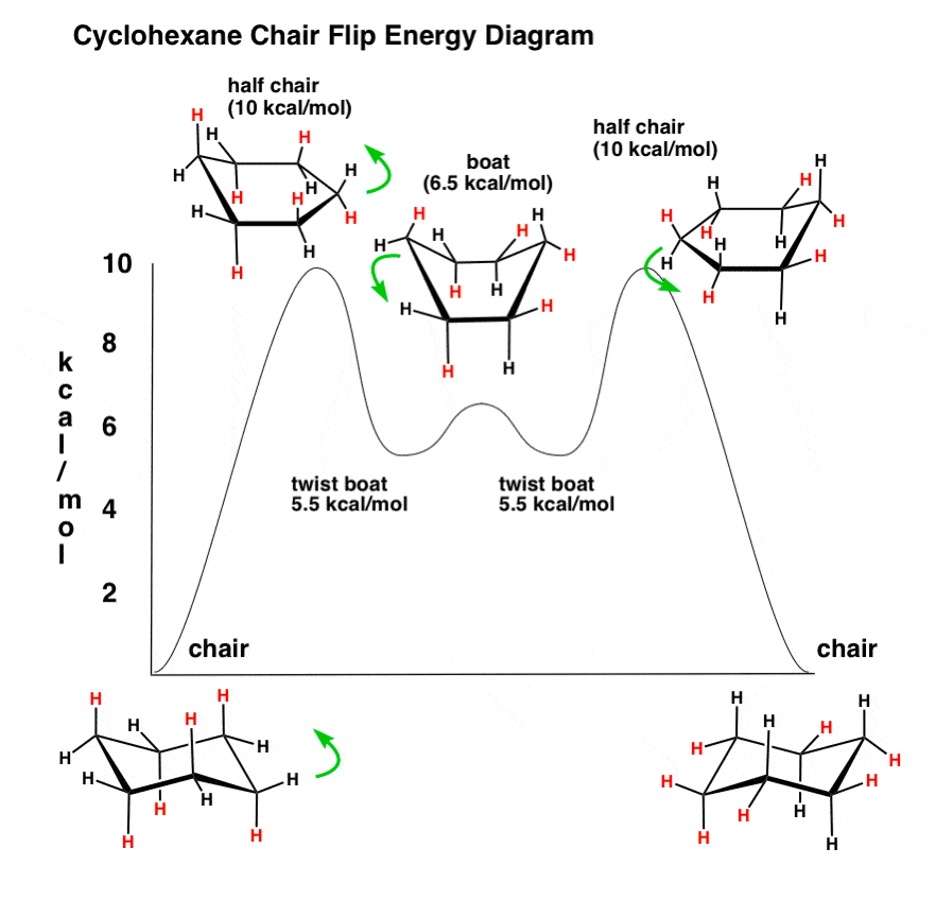
Figure 3: The Different Conformations of Cyclohexane3
When looking at 1,2-dimethylcyclohexane, we can have the trans version (subsituents are on opposite sides) or the cis version (substituents are on the same side).
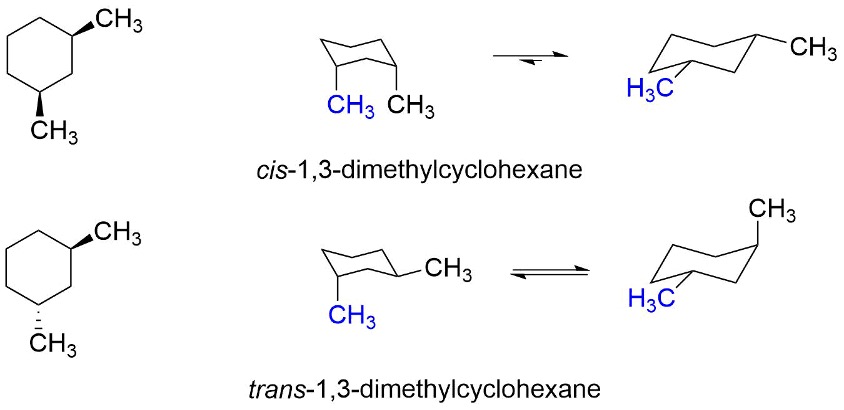
Figure 4: 1,2-dimethylcyclohexane4
Generally, bigger substituents that are placed equatorially make for more stable molecules.
References
Petrucci RH, Herring FG, Madura JD, Bissonnette C. General Chemistry: Principles And Modern Applications. 11th ed. Toronto, ON: Pearson Canada; 2017:1215-1228.
Ashenhurst J. Newman projection of butane (and gauche conformation). Master Organic Chemistry. https://www.masterorganicchemistry.com/2020/05/29/newman-projection-of-butane-and-gauche-conformation/. Published October 22, 2021. Accessed June 29, 2022.
Ashenhurst J. The cyclohexane chair flip - energy diagram. Master Organic Chemistry. https://www.masterorganicchemistry.com/2014/06/06/the-cyclohexane-chair-flip-energy-diagram/. Published August 4, 2020. Accessed June 29, 2022.
Libretexts. 3.9: Conformations of disubstituted cyclohexanes. Chemistry LibreTexts. https://chem.libretexts.org/Courses/Siena_Heights_University/SHU_Organic_Chemistry_I/3%3A_Chapter_3_Conformations_and_Cycloalkanes/3.09%3A_Conformations_of_Disubstituted_Cyclohexanes. Published August 11, 2020. Accessed June 29, 2022.